Abstract
Background:
Tumor burden in multiple myeloma (MM) is routinely evaluated in the bone marrow, though its prognostic value is not proven. There is an increasing interest in liquid biopsies due to its minimally invasive nature and more comprehensive evaluation of tumor burden. Growing evidence supports the quantification of circulating plasma cells (cPCs) measured by multiparameter flow cytometry (MFC) as a powerful diagnostic biomarker suitable for risk stratification of newly diagnosed transplant eligible MM (Garces et al, EHA 2021). Nevertheless, there are virtually no data regarding prognostic impact of cPCs in MM patients ineligible for transplantation. Primary plasma cell leukemia (pPCL) is a rare and most aggressive monoclonal gammopathy with dismal outcomes defined by more than 20% of cPCs and/or absolute cPCs count of ≥2 x10 9/L. Recently, there have been many efforts to redefine these criteria as lower number of cPCs probably portends equally poor prognosis.
Aims:
To evaluate prognostic significance of cPCs in a large cohort of transplant ineligible (Tx-ineligible) newly diagnosed MM patients and to define cut-offs for risk stratification. Moreover, to establish cut-off identifying ultra high risk MM patients mimicking the prognosis of pPCL and propose new definition of pPCL.
Methods:
Circulating PCs were measured by 8 color flow cytometry in 402 Tx-ineligible MM patients (including n=7 pPCL) diagnosed between 2012 and 2019 at University Hospitals Brno and Ostrava, Czech Republic. Patients were treated in real-world setting and the clinical analysis was performed retrospectively based on data from the Czech Registry of Monoclonal Gammopathies. Median follow-up was 20.5 months. The intermediate cutoff was identified using ROC analysis considering overall survival (OS). Moreover, data from the large published cohort of pPCL patients treated in real-world setting were used to find a new cut-off identifying these ultra-high-risk pPCL-like patients (Jurczyszyn et al. BJH,2018).
Results:
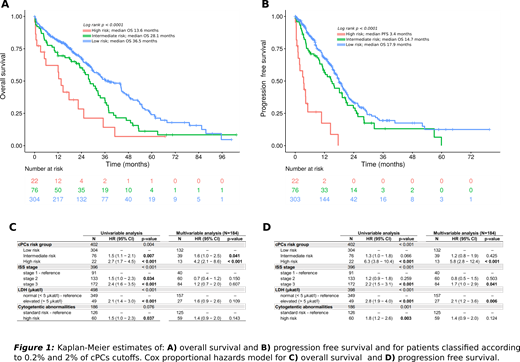
Circulating PCs were detected in peripheral blood (PB) of 303/402 (75%) patients. In 303 patients with detectable cPCs the median percentage was 0.06% with range 0.0008% - 79%. The median limit of detection of MFC technique was 0.006 (sensitivity 10e-5). Patients stratification into 3 subgroups according to quantification of cPCs (low: ≤ 0.2%; intermediate: 0.2 - 2% and high: >2.0%) resulted in significantly different OS (36.5 vs. 28.1 and 13.6 months; p < 0.0001) and progression free survival (PFS) (17.9 vs. 14.7 and 3.4 months; p < 0.0001). Patients with no detected cPCs (0%) did not separate from subgroup >0% to <0.2% (p >0.05) suggesting that next-generation flow cytometry (NGF) with sensitivity 10e-6 is needed for the identification of this favorable prognostic group. In order to demonstrate that patients with more than 2% of cPCs have similarly poor outcome as pPCL patients, we compared those with 2% - 20% (n=15) vs. those with >20% (n=7) of cPCs. The outcomes were practically identical with median PFS of 3.1 vs. 4.2 months (p = 0.23) and median OS of 13.6 vs. 14.6 months (p=0.23).
Next, we analyzed patient´s characteristics in association with the level of cPCs (low, intermediate and high) and we demonstrated that there was significantly higher proportion of patients with ISS III stage (38%, 52% and 82%), elevated LDH level (8%, 19% and 55%) and high risk cytogenetics (12%, 21% and 36%) hand in hand with increasing number of cPCs. CPCs were identified as the most powerful prognostic marker in univariate (HR=2.7 for OS and HR=6.3 for PFS; p=0.001) and multivariate analysis (HR=4.2 for OS and HR=5.8 for PFS; p<0.001), including ISS, LDH and FISH cytogenetics.
Conclusion:
The quantification of cPCs in PB of newly diagnosed MM is the most powerful prognostic factor as we demonstrated on a large cohort of transplant ineligible patients. We defined 2% of cPCs as a new cut-off for ultra high risk myeloma resembling behavior of primary PCL. We propose this 2% cut-off for redefinition of pPCL criteria that warrants further investigation in prospective setting. To identify subgroup with especially favorable outcome with no detectable cPCs, NGF with high sensitivity of 10e-6 is needed. Quantification of cPCs by MFC is easy, fast, affordable and worldwide available procedure providing highly relevant prognostic information that might be implemented into routine clinical practice.
Jurczyszyn: Janssen-Cilag, Amgen: Honoraria, Speakers Bureau. Castillo: Abbvie: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Janssen: Consultancy; Roche: Consultancy; TG Therapeutics: Research Funding. Hajek: Janssen: Consultancy, Honoraria, Research Funding; Pharma MAR: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal